Bravecto® Spot On

A Spot-On product for the treatment and prevention of tick, flea and mite infestations in dogs and cats.

Download the Bravecto® Spot-On owners brochure below.

FOR ANIMAL USE ONLY
BRAVECTO® SPOT-ON
Reg. No. G4292 (Act 36/1947)
This product may only be sold by persons registered in terms of section 23 (1) (c) of the Veterinary and Para-Veterinary Professions Act, 1982 (Act No. 19 of 1982).
INDICATIONS
For the treatment and prevention of tick, flea and mite infestations in dogs and cats.
Bravecto® Spot-On can be used as part of a treatment strategy for Flea Allergy Dermatitis (FAD). For the treatment of demodicosis caused by sarcoptic mange mites in dogs. For the treatment of Otodectes spp. mite infestations in dogs and cats.
In cats, Bravecto® Spot-On provides immediate and persistent flea and tick activity for 3 months.
In dogs, Bravecto® Spot-On provides immediate and persistent tick activity for 4 months and flea activity for 6 months. Bravecto® Spot-On is well tolerated in MDR1 dogs.COMPOSITION
Each 1 mℓ of Bravecto® Spot-On contains 280 mg fluralaner.
Each pipette delivers:
| Miniature dogs: | 112,5 mg fluralaner | Small cats: | 112,5 mg fluralaner | |
| Small dogs: | 250 mg fluralaner | Medium cats: | 250 mg fluralaner | |
| Medium dogs: | 500 mg fluralaner | Large cats: | 500 mg fluralaner | |
| Large dogs: | 1 000 mg fluralaner | |||
| Extra-large dogs: | 1 400 mg fluralaner |
CLINICAL PHARMACOLOGY
Peak fluralaner concentrations are achieved between 7 and 42 days following topical administration and the elimination half-life ranges between 14 and 29 days. The bioavailability of fluralaner following oral and topical administration is approximately 25 %.
PHARMACODYNAMIC PROPERTIES
Fluralaner is an acaricide and insecticide. It is efficacious against ticks, fleas and mites.
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives.
Fluralaner is an inhibitor of parts of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
CAUTION
STORAGE
Store at or below 30 °C in a cool, dry place.WARNINGS
- Handle with caution.
- For cats, safety has not been established during pregnancy and lactation. Use only according to the benefit/risk assessment by the responsible veterinarian.
- Do not use in the case of hypersensitivity to the active substance or to any of the excipients.
- Parasites need to start feeding on the host to become exposed to fluralaner; therefore the risk of the transmission of parasite-borne diseases cannot be excluded, but is reduced by the speed of efficacy.
- Care should be taken to avoid contact with the eyes of the animal.
- Do not use directly on skin lesions.
- Dogs can be washed/shampooed 3 days after treatment.
- Safety in puppies less than 8 weeks old and/or dogs weighing less than 2 kg has not been established.
- Safety in kittens less than 11 weeks old and/or cats weighing less than 1,2 kg has not been established.
- The product should not be administered at intervals shorter than 8 weeks as the safety for shorter intervals has not been tested.
- Keep the product in the original packaging until use.
- Do not have contact with the animal or allow children to have contact with the animal until the application site is dry.
- The product is highly flammable. Keep away from heat, sparks, open flames or other sources of ignition.
- KEEP OUT OF REACH OF CHILDREN, UNINFORMED PERSONS AND ANIMALS.
- Although this remedy has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
PRECAUTIONS
- For topical use only. Avoid oral ingestion.
- Do not eat, drink or smoke while handling the product.
- Wash hands thoroughly with soap and water immediately after the product has been used.
- Do not store with food, drinks, medication or household products.
- Medicines should not be disposed of via wastewater or household waste but in accordance with the National Environmental Management: Waste Act, 2008 (Act No. 59 of 2008). These measures should help to protect the environment.
INTERACTIONS
Fluralaner is highly bound to plasma proteins and might compete with other highly bound drugs such as non-steroidal anti-inflammatory drugs (NSAID’s) and the coumarin derivative warfarin. Incubation of fluralaner in the presence of carprofen or warfarin in dog plasma at maximum expected plasma concentrations did not reduce the protein binding of fluralaner, carprofen or warfarin.
During laboratory and clinical field testing, no interactions between Bravecto® Spot-On and routinely used veterinary medicinal products were observed.
ADVERSE REACTIONS
Commonly observed adverse reactions in clinical trials were mild and transient skin reactions at the application site (1,2 % of treated dogs and 2,2 % of treated cats). Cat’s skin reactions were such as erythema and pruritus (most likely due to a transient discomfort) or alopecia (secondary effect due to scratching or licking).
As a result of transient discomfort shortly after administration, the following signs were observed: apathy/tremors/anorexia (0,9 % of treated cats) or vomiting/hypersalivation (0,4 % of treated cats).
If you notice any serious effects or other effects not mentioned in this package leaflet, please inform your veterinarian.
DIRECTIONS FOR USE – USE ONLY AS DIRECTED
Bravecto® Spot-On should be administered in accordance with the following table (corresponding to a dose of between 25 and 56 mg fluralaner/kg body weight in dogs and between 40 and 94 mg fluralaner/kg body weight in cats within one weight band):
| Weight of dog/cat (kg) | Pipette size to be used | Volume (mℓ) | Fluralaner (mg) |
| 2 – 4,5 | Miniature dogs | 0,4 | 112,5 |
| > 4,5 – 10 | Small dogs | 0,89 | 250 |
| > 10 – 20 | Medium dogs | 1,79 | 500 |
| > 20 – 40 | Large dogs | 3,57 | 1 000 |
| > 40 – 56 | Extra-large dogs | 5,0 | 1 400 |
| > 56 | The appropriate combination of pipettes should be used. | ||
| 1,2 – 2,8 | Small cats | 0,4 | 112,5 |
| > 2,8 – 6,25 | Medium cats | 0,89 | 250 |
| > 6,25 – 12,5 | Large cats | 1,79 | 500 |
For optimal control of tick infestation, Bravecto® Spot-On should be administered every 3 months for cats and every 4 months for dogs.
For optimal control of flea infestation, Bravecto® Spot-On should be administered every 3 months for cats and every 6 months for dogs. Within each weight range a whole pipette must be used.
For dogs weighing more than 56 kg, use a combination of 2 pipettes that most closely matches the body weight.
For cats weighing more than 12,5 kg, use a combination of 2 pipettes that most closely matches the body weight.Method of administration
Step 1:
Immediately before use, open the sachet and remove the pipette. The pipette should be held by the upper part of the tail base (crimped end) or by the upper rigid portion below the cap in an upright position (tip up) before opening it. The cap should be rotated clockwise or counter clockwise one full turn. The cap will stay on the pipette; it should not be removed. The pipette is open and ready for application when the breaking of the seal is felt.
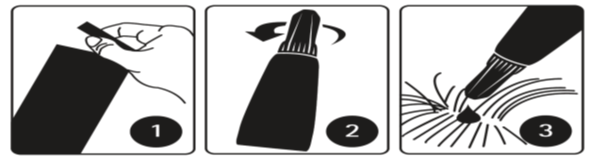
Step 2:
The dog/cat should be standing or lying with its back horizontally during application. Part the fur at the administration site. Place the pipette tip vertically against the skin between the shoulder blades of the dog or pipette tip on the base of the skull of the cat.
Step 3:
Dogs:
Squeeze the pipette gently and apply the entire contents directly to the dog’s skin in one (when volume is small) or several spots along the dog’s dorsal line from the shoulder to the base of the tail. It should be avoided to apply an excessive amount of solution at any one spot that could cause some of the solution to run or drip off the dog.
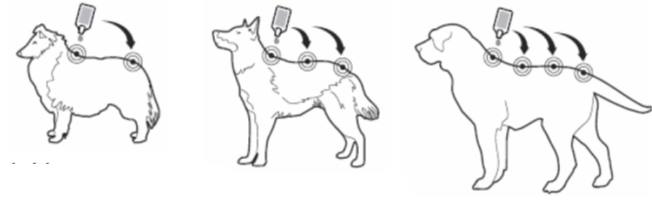
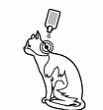
Cats:
Squeeze the pipette gently and apply the entire contents directly to the cat’s skin. The product should be applied in one spot (cats up to 6,25 kg body weight) or two spots (cats weighing more than 6,25 kg body weight). If two spots are needed, the first spot should be applied at the base of the skull and the second one between the shoulder blades.
It is important to deliver the solution onto an area that cannot be easily licked by the cat. As cats are fastidious groomers, they may inadvertently ingest product if easily reached. Ingestion may cause adverse reactions, and will remove product from the coat. If multiple cats are treated and may mutually groom, it is recommended that cats are separated while product dries.
OVERDOSE
Dogs:
Safety was demonstrated in puppies aged between 8 and 9 weeks and weighing between 2,0 and 3,7 kg treated with overdoses of up to 5 times the maximum recommended dose on three occasions at shorter intervals than recommended (8-week intervals).
Safety was demonstrated in breeding, pregnant and lactating dogs treated with overdoses of up to 3 times the maximum recommended dose.
The product was well tolerated in collies with a deficient multidrug-resistance-protein 1 (MDR1 -/-) following single oral administration at 3 times the recommended dose.
Cats:
Safety was demonstrated in kittens aged between 11 and 13 weeks and weighing between 1,2 and 1,5 kg treated with overdoses of up to 5 times the maximum recommended dose on three occasions at shorter intervals than recommended (8-week intervals).
Oral uptake of the product at the maximum recommended dose was, both locally and systemically, well tolerated in cats, apart from some self-limiting salivation and coughing immediately after administration.
In cats, safety has not been established during pregnancy and lactation.
EFFICACY
Bravecto® Spot-On was shown to be effective against ticks, fleas and mites.
It is a systemic insecticide and acaricide.
Cats: the product provides immediate and persistent flea and tick activity for 3 months.
Dogs: the product provides immediate and persistent tick activity for 4 months and flea activity for 6 months.
Ticks and fleas must attach to the host and commence feeding in order to be exposed to the active substance. The onset of effect is within 8 hours.
Bravecto® Spot-On is efficacious for the treatment of the following:
Ticks
Dogs: Ixodes ricinus, Rhipicephalus sanguineus, Haemaphysalis elliptica and Dermacentor reticulatus.
Cats: Ixodes ricinus and Haemaphysalis elliptica.
Directly after treatment, at least 90 % of ticks on the animals are killed within 8 hours. During the whole treatment interval, at least 90 % of ticks on the animals are killed within 12 hours.
Fleas
Dogs: Demodicosis caused by Demodex spp. and Sarcoptic mange mites and Otodectes spp. mite infestations.
Cats: Ctenocephalides felis.
Directly after treatment, at least 95 % of fleas are killed within 8 hours.
During the whole treatment interval, at least 95 % of fleas on dogs are killed within 12 hours.
Mites
Dogs: Mange caused by Demodex spp. and Sarcoptic mange mites. For the treatment of Otodectes spp. mite infestations.
Cats: Otodectes spp.
Effect on immature stages
The product kills adult as well as juvenile ticks (larvae, nymphs). Newly emerged fleas on animals are killed before viable eggs are produced. An in vitro study also demonstrated that very low concentrations of fluralaner stop the production of viable eggs by fleas.
Control of flea infestations and Flea Allergy Dermatitis (FAD)
The flea life cycle is broken due to the rapid onset action and long-lasting efficacy against adult fleas on the animal and the absence of viable egg production. Bravecto® Spot-On effectively controls environmental flea populations in areas to which the animals have access.
PRESENTATION
Unit dose pipette made of laminated aluminium/polypropylene foil containing a colourless to yellowish solution, practically free from visible particles closed with cap and packed in a sachet.
Each carton box contains 1 or 2 pipettes.
| REGISTRATION HOLDER Intervet South Africa (Pty) Ltd. 20 Spartan Road, Spartan 1619, RSA Tel: +27 (0) 11 923 9300 Fax: +27 (0) 11 392 3158 www.msd-animal-health.co.za | MANUFACTURER Patheon Manufacturing Services LLC 5900 Martin Luther King Jr. Highway Greenville, North Carolina (NC) 27834 United States of America |
DATE OF PUBLICATION OF THIS PACKAGE INSERT
10 October 2018

