Revalor® G
A slow release anabolic agent containing trenbolone acetate and oestradiol which increases rate of mass gain in feedlot cattle (bulls, feeder steers and heifers)
FOR ANIMAL USE ONLY
REVALOR® G
Reg. No. G2714 (Act 36/1947)
INDICATIONS
For cattle
A slow release anabolic agent containing trenbolone acetate and oestradiol which increases the rate of mass gain in cattle (bulls, feeder steers and heifers).
COMPOSITION
Each dose is equivalent to 40 mg trenbolone acetate and 8 mg oestradiol
(2 small yellow pellets, each containing 20 mg of trenbolone acetate and 4 mg oestradiol).
STORAGE
- Store unopened product below 25 °C.
- Avoid excessive heat and humidity.
- Use product before the expiry date.
WARNINGS
- NOT TO BE USED IN ANIMALS INTENDED FOR SUBSEQUENT BREEDING, OR IN DAIRY ANIMALS.
- Implant pellets in the back of the ear only.
- Do not salvage the implanted site for human or animal consumption.
- Store away from food and feed.
- Dispose of any empty containers/cartridges in accordance with local waste disposal regulations and do not re-use for any other purpose.
- KEEP OUT OF REACH OF CHILDREN, UNINFORMED PERSONS AND ANIMALS.
- Although this remedy has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
DIRECTIONS FOR USE – USE ONLY AS DIRECTED.
Dosage
One implant containing 40 mg trenbolone acetate and 8 mg oestradiol is administered to each animal. The 2 pellets which make up the dosage of Revalor® G are contained in one division of the multiple dose cartridges. There are ten doses in each cartridge.
Site of implantation
After appropriately restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site.
The site of implantation is the soft skin on the posterior aspect of the ear.
The implant should be placed as far from the head as possible without inserting the implanter into the area where the skin is firmly attached to the cartilage.
THE IMPLANT MUST NOT BE PLACED CLOSER TO THE HEAD THAN THE EDGE OF THE CARTILAGE RING FARTHEST FROM THE HEAD.
The location of insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site.
Care should be taken to avoid injuring the major blood vessels or cartilage of the ear.
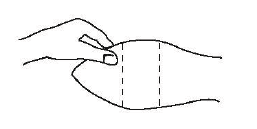
Fig. 1 – Ear of bovine ready for implantation 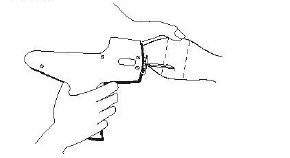
Fig. 2 – Rear view of the bovine ear showing the site for insertion of the implanter needle
PRESENTATION
Contains 100 doses in 10 cartridges with 10 implants per cartridge.
REGISTRATION HOLDER
Intervet South Africa (Pty) Ltd.
20 Spartan Road, Spartan
1619, RSA
Tel: +27 (0) 11 923 9300
Fax: +27 (0) 11 392 3158
www.msd-animal-health.co.za
MANUFACTURER
Intervet GesmbH
Siemensstrasse 105
A-1210 Vienna
Austria
DATE OF PUBLICATION OF PACKAGE INSERT
2 March 2007
